What Term Is Typically Used to Describe Ionic Solids
To understand and recognize covalent-network solids ionic solids and metals. Poroelasticity is the term used to describe the interaction between fluid flow and solids deformation within a porous medium.
Physical Properties Of Ionic Compounds Read Chemistry Ck 12 Foundation
Other possible packing arrangements of atoms in solids include simple cubic and body-centered cubic BCC.

. In chemistry an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. Melting point and freezing point are the two terms used to describe the temperature at. In some materials neighboring atoms actually move away from a vacancy because they can form better bonds with atoms in the other directions.
Ionic crystals are extremely stable because considerable energy is required to break ionic bonds. Solubility constants are used to describe saturated solutions of ionic compounds of relatively low solubility see solubility equilibrium. For all FCC ionic solids alpha is approximately 175.
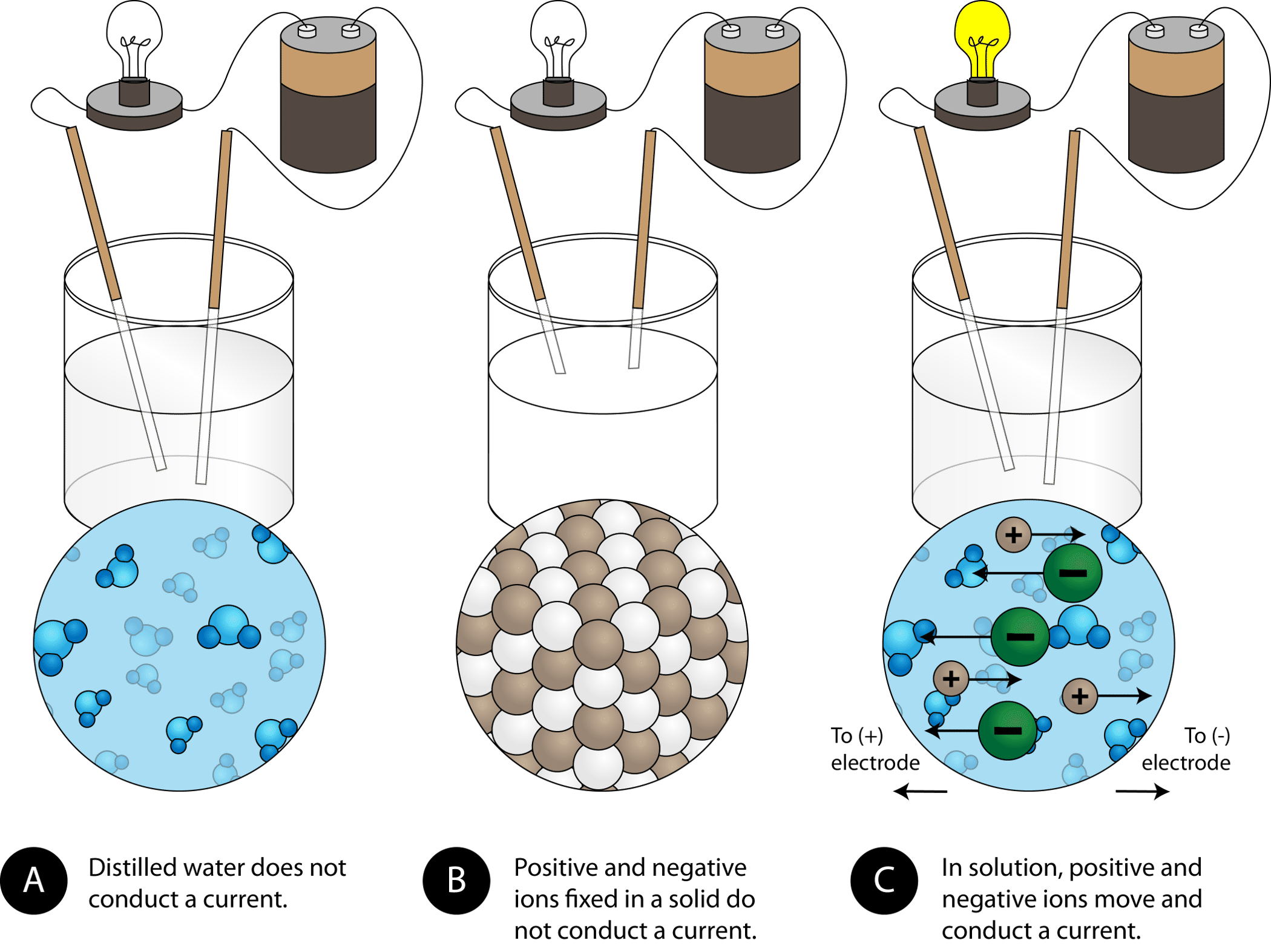
Describe the metallic bond as the electrostatic attraction between a lattice of positive ions and delocalized electrons. Solid composed of positive and negative ions held together by strong electrostatic attractions metallic solid. A vacancy or pair of vacancies in an ionic solid is sometimes called a Schottky defect.
Ionic solids typically have high melting points and boiling points. The term ____ is not typically used to describe ionic solids due to the absence of discrete units in their crystal lattice structure. Chemists use the term intermolecular forces to describe the attractions between two or more molecules In solids however it is difficult to distinguish one molecule from another since the forces are the same throughout.
ANSWER NOW 80 POINTS. The smallest repeating pattern of crystalline solids is known as the unit cell and unit cells are like. 2 Al HCI 2 AICI 3 H unbalanced Describe in your own words a situation where chemical energy is transformed to potential energy.
Chemists usually apply the term ionic solid to binary compounds of the metallic elements of Groups 1-2 with one of the halogen elements or oxygen. Ionic solids containing OH ions are termed as basic compounds because they release OH ions it increase the pH. Electricity Magnetism and Light 2002.
Ionic solids form when electrostatic attraction causes anions and cations to form a crystal lattice. Molecule If energy is absorbed in a chemical reaction then it appears on the ___ side of the reaction arrow in the chemical equation. Ionic solids containing H ions are termed as acidic compounds because these solids release H ions when dissolved in water it reduces the pH of the aqueous medium.
Attractive Forces within Solids Learning Goal. This type of material is typically associated with natural objects such as rocks and solids as well as biological tissues foams ceramics and paper. Distant ions make a significant contribution to this sum so it converges slowly and many terms must be used to calculate alpha accurately.
As their name indicates porous materials are solid structures comprised of pores or voids. Type your answer inside the box. Define the basic terms relating to the structure of solids such as crystalline solid amorphous solid lattice ionic ionic solid molecular solid atomic solid metallic solid and network solid.
At an atomic level an ionic crystal is a regular structure with the cation and anion alternating with each other and forming a three-dimensional structure. Solid composed of metal atoms molecular solid. Although molecular compounds form crystals they frequently take other forms plus molecular crystals typically are softer than ionic crystals.
Since it is a product of ion concentrations in equilibrium it is also known as the solubility product. Ionic solids are characterized by high melting points. The solubility constant is a special case of an equilibrium constant.
These can be simple ions such as the sodium and chloride in sodium chloride or polyatomic species such as. Chemists usually apply the term ionic solid to binary compounds of the metallic elements of Groups 1-2 with one of the halogen elements or oxygen. Ionic compounds form crystal lattices rather than amorphous solids.
In an ionic crystal each ion is surrounded by ions with an opposite charge. In ionic solidssuch as saltand in ionic fluids or electrolytessuch as salt water or battery acidthe charge carriers are ions producing ionic conduction. What term is used to describe splitting a large atomic nucleus into two smaller ones.
There are two main categories of solidscrystalline solids and amorphous solids. If 54 g Al are reacted with excess HCI how many moles of H2 will be produced. Interstitials are atoms which occupy a site in the crystal structure at which there is usually not an atom.
Crystalline solids are those in which the atoms ions or molecules that make up the solid exist in a regular well-defined arrangement. In an ionic solid the bonds are electrostatic and the break up. It describes the balance between.
Solid composed of neutral molecules held together by intermolecular forces of attraction. The compound is neutral overall but consists of positively charged ions called cations and negatively charged ions called anions. 5 points Health Grade 3 Quiz.

Ionic Solids Molecular Solids Metallic Solids Network Covalent Solids Atomic Solids Youtube

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

Question Video Explaining The Difference Between Metals And Ionic Solids Nagwa
No comments for "What Term Is Typically Used to Describe Ionic Solids"
Post a Comment